
Dever de casa para os peritos traduzirem (Fonte: Chemistry World)
The scientists on the inside of advanced fingerprinting research are cross-examined by Simon Hadlington
Since the late 1800s, fingerprints have formed the central pillar of forensic science, exploiting the fact that no two individuals’ prints are identical. Fingerprints, or fingermarks, are made when the tip of the finger comes into contact with a surface and leaves an impression of the ridges. These ridges contain secretions of sweat from eccrine glands in the fingertip.
Visible fingerprints are enhanced by dusting with a powder – typically flaked aluminium – that sticks to the eccrine gland residues. Invisible prints – termed latent – can be developed by a number of techniques that usually rely on a chemical reaction with a molecular component within the fingerprint.
|
Latent fingerprints on spent cartridge cases can be enhanced using a large electrical potential and a conducting powder
|
However, often the prints are difficult to develop, depending on their age or the surface upon which they have been deposited, and forensic scientists are constantly looking for new and better methods to enhance them. Researchers are also finding ways to interrogate the molecular composition of the residues within fingerprints to discover information about the person who deposited them – whether they have handled or consumed particular drugs, for example.
Bang, bang, bang
John Bond, at the University of Leicester, UK, worked for many years as a forensic scientist for the police force. ‘There are a number of techniques which are widely used across the world to develop latent fingerprints on surfaces, and these all work by a chemical or physical reaction with the products of sweat,’ says Bond. ‘The technique depends largely on whether the substrate is porous (such as paper) or non-porous (such as metal or glass) and what has happened to the print before it gets to be processed.’

|
Latent fingerprints on spent cartridge cases can be enhanced using a large electrical potential and a conducting powder
© UNIVERSITY OF LEICESTER
|
The main components of fingermarks are water, amino acids, salts and fatty acids. The standard test for latent marks on a porous substrate is to expose it to a solution of ninhydrin (2,2-dihydroxyindane-1,3-dione) which reacts with the terminal amines of lysine residues within the sweat to create a purple product. For non-porous substrates the most popular method is to fume the sample with cyanoacrylate – superglue – vapour. This polymerises – for reasons that are not fully understood – when it comes into contact with the fingermark, becoming visible. But these methods do not always work.
One example of a ‘difficult’ surface is spent ammunition cartridges or shells. ‘Ordinarily for a fresh print on metal, superglue works very well,’ says Bond. ‘But on a spent shotgun cartridge casing it is very difficult to recover sweat using conventional techniques. The main reason is that the extremely high temperatures that the shells are exposed to vaporises the sweat.’
Rather than attempt to enhance marks from whatever sweat might remain, Bond has been examining the pattern of corrosion left on the metal where the fingers have handled the ammunition. When someone touches the brass of a bullet shell or shotgun cartridge, the salts in their sweat will cause the surface to corrode. ‘The amount of corrosion depends on the amount of salt in the person’s sweat and this varies between individuals,’ explains Bond. Another factor is the composition of the brass layer at the site of corrosion – whether it is predominantly copper, zinc or a mixture of the two. Bond has shown that in some cases the marks can be made visible by a clever trick of semiconductor physics.
‘We have discovered that in brass, the corroded layer of either zinc or copper oxide that is in contact with the underlying metal forms what is known as a Shottky barrier – an interface between a semiconductor, which the oxides are, and a conductor.’ If a large electrical potential is applied to the sample, a potential difference develops between the two surfaces, with the oxide layer acting as an insulator. If a fine metal powder is then applied to the surface of the sample, it preferentially sticks to the area of lower potential – the corroded part.
‘It does not work every time,’ concedes Bond. ‘It depends on the corrosion product – it needs to be mainly one oxide or the other, rather than a mixture of the two.’ The system is nevertheless sufficiently useful to have just been commercialised.
I’m fuming

|
Disulfur dinitride vapour polymerises along the ridges of latent fingermarks, making them more visible
© PAUL KELLY/ROB KING
|
One of the most exciting new prospects in latent fingermark enhancement was discovered by accident in Paul Kelly’s lab at Loughborough University in the UK. ‘We were looking at the polymerisation of disulfur dinitride [S2N2] in the pores of zeolites when we noticed that when exposed to the vapour, fingermarks on the glassware we were using would become developed and turn a blue-black colour characteristic of the polymer.’ Kelly had recently read a report on how the enhancement of fingerprints was still proving challenging in a number of circumstances. ‘A lightbulb lit up in my head,’ he recalls.
It soon became clear that S2N2 would polymerise on latent fingermarks on a range of surfaces, even when the marks had been thoroughly washed, as well as on conventionally difficult surfaces such as spent shotgun cartridges. Markings from substances such as inks that have been thoroughly washed away from metal surfaces can also be developed using S2N2 vapour. ‘Forensic people are very interested in this because it is a fuming technique, and so relatively large samples can be tested quickly,’ Kelly notes. As is the case for superglue, it is not yet clear why S2N2 vapour polymerises along the ridges of marks.
Turning to fat
Meanwhile, Daniel Mandler and Joseph Almog at the Hebrew University of Jerusalem, Israel, are working to improve enhancement on porous surfaces, such as paper, that have been wetted. Wetting normally washes much of the amino acid content of the mark away.
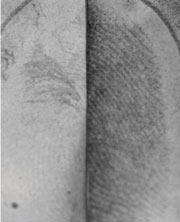
|
Visualising prints on paper that has been wet is problematic. The print on the left was treated with a typical silver-based developer. Gold nanoparticles were applied to the print on right before the silver developer was added
|
‘The classical approach is for a certain number of reagent molecules to react with a fixed number of substrate molecules [in the fingerprint], such as amino acids,’ says Almog. ‘For extremely small amounts of substrate this approach will not be sensitive enough.’ Instead, says Almog, ways to amplify the signal from the small amounts of material left in the fingermark need to be developed. ‘After the amino acids have gone, what remains on the sample is mainly oily residues,’ Mandler explains. ‘We reasoned that it may be possible to incorporate metallic nanoparticles into these fatty deposits if we stabilised the nanoparticles with hydrophobic molecules.’
The team soaked fingermarked samples in a solution containing gold nanoparticles that had been rendered hydrophobic by the attachment of alkanethiol chains. They found that the nanoparticles accumulated almost exclusively on the ridges of the fingermarks, where the fats reside. The nanoparticles then catalysed the deposition of silver from a standard silver colloid solution used for developing latent fingerprints. The end result is a visible fingerprint. The researchers are now experimenting with different components and reaction conditions to improve the system further.
Ace antibodies
Xanthe Spindler at the University of Technology, Sydney in Australia is also utilising the power of nanoparticles to enhance latent fingermarks. She has taken antibodies against the amino acids found in marks and attached them to gold nanoparticles. When exposed to a latent mark, the antibodies target and attach themselves to any amino acids present. A second set of antibodies, this time targeting the nanoparticle-bound antibodies, is then applied. These second antibodies contain a red fluorescent dye that can be used to develop a high-quality image of the fingermark.
Spindler has found that her system seems especially effective for fingerprints that were made some time ago. ‘At the moment we’ve only tested up to a fingerprint age of one year, but the results indicated there is scope to investigate its use on older fingerprint samples that typically give poor results using conventional techniques,’ says Spindler. ‘The research is still in the early phases, so there are still a few kinks to work out in the application procedure before we’ll start to see a definite benefit compared to traditional methods.’
Digging Deep
At the University of Lincoln in the UK, Ruth Croxton is studying the components of fingermarks and what happens to the chemistry of the mark when it is laid down. She hopes that a deeper understanding of the chemical composition of marks may open the way to new and better enhancement methods.
‘For some of the techniques, we are not entirely sure why they work,’ says Croxton. ‘It would be nice to have an enhancement technique that always worked: we want to understand what is there, what goes on, what happens when the fingerprint ages? Can we find something that is always there in reasonable quantity?’
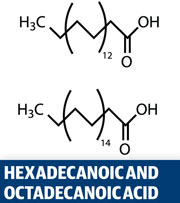
Croxton’s lab has carried out comprehensive gas chromatography-mass spectrometry studies of latent marks, identifying and quantifying the range of components. They found that the most abundant fatty acids are hexadecanoic acid, octadecanoic acid and cis-9-octadecenoic acid. ‘There was a wide variation in the relative amounts of each of these in the samples,’ Croxton says. They found that that the amount of fatty acid present increased if the face was touched before the fingermark was deposited – because the face is rich in an oily secretion called sebum. The triterpene squalene was also present in all samples. The most abundant amino acid identified was serine, followed by glycine, alanine and aspartic acid.
‘There is a wide variation in the composition of fingermarks depending on things such as whether the hands had been recently washed or the face had been touched a lot,’ says Croxton. ‘What we want to do is improve methods for identifying the various components and also to look at other substances in the fingermark. Hopefully this will help us to understand how the various enhancement systems work at the molecular level.’
Print interrogation
As well as providing information about an individual’s identity, fingermarks can also reveal information about the person who left them – whether he or she had consumed or handled drugs or other substances, for example. ‘If a fingerprint is not present on the police database to provide a match, its evidential power is limited,’ explains Simona Francese at Sheffield Hallam University in the UK. ‘We want to use and develop technology that will enable us to add intelligence in the case under investigation.’
Francese’s team is using and refining a technique called MALDI-MSI (matrix-assisted laser desorption/ionisation-mass spectrometry imaging) to interrogate fingerprints for their chemical content. The system produces both an enhanced image of the pattern of the fingerprint ridges, as well as detecting various chemical species that are present in the print. Multiple ions can be detected in a single analysis, and the print is not damaged by the technique.
The researchers are able to detect a wide range of molecules, including drugs and caffeine from ingested coffee, Francese says. The power of the method has been neatly illustrated with a study on condom lubricants. ‘In many cases of sexual assault, the perpetrator uses a condom for protection and to avoid leaving DNA evidence,’ says Francese. ‘We have carried out experiments looking at condom lubricant residues in fingermarks, and our method can detect a variety of lubricants, such as PDMS [polydimethylsiloxane] and PEG [polyethylene glycol]. Different brands of condom have different lubricant profiles, so the technique could be used to identify a particular brand of condom that was used.’ The team is now further refining the technique and drawing up protocols for its use in forensic laboratories.
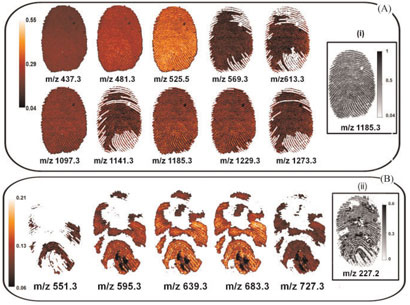
|
Condom lubricants can be detected in fingerprints using mass spectrometry imaging. Section A shows images of a print after touching Condomi Max Love condoms, and section B Trojan Enz
© WILEY
|
Branching out
At the University of East Anglia in the UK, David Russell has pioneered the use of antibodies to detect metabolites – from drugs, for example – in sweat excretions in fingerprints. While this has the potential to provide information about a suspect from prints at the scene of a crime, a more immediate application is as a portable and rapid drug-testing device.
A company, Intelligent Fingerprinting, has been spun out from the research and has just launched a prototype fingerprint drug-testing kit. The firm’s business development manager Paul Yates explains how the system works. ‘We use nanoparticles of gold or iron oxide that are coated with the antibody of interest – a metabolite of cocaine, for example – together with a fluorescent dye. When the subject’s fingerprint is exposed to the nanoparticles, if any of the metabolite is present in the sweat of the fingerprint the antibodies will bind and can be detected by the fluorescence.’
The person being tested makes a fingerprint impression on a cassette containing the antibody system, which is then inserted into an electronic ‘reader’. This takes an optical image of the fingerprint – important for ensuring the correct identity of the person being tested – as well as a readout of the potential presence of drugs. The prototype is configured to detect a single drug metabolite, but, says Yates, it should be relatively straightforward to enable multiple metabolites to be tested simultaneously. Yates says that the device could have wide applications, from roadside drug-testing, similar to a breathalyser for alcohol testing, to testing athletes for banned substances.
Simon Hadlington is a science writer based in York, UK
 Appes.com.br Associação dos Peritos Papiloscópicos do Estado do Espirito Santo
Appes.com.br Associação dos Peritos Papiloscópicos do Estado do Espirito Santo


